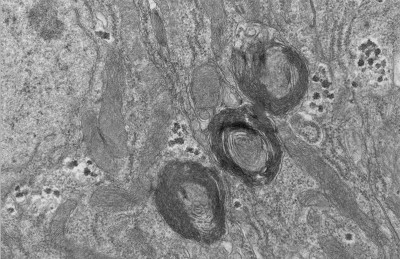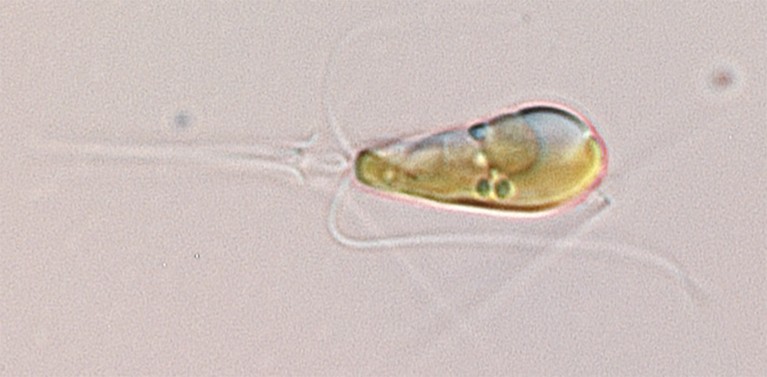
A Braarudosphaera bigelowii cell magnified 1,000-fold.Credit: Tyler Coale
Researchers have discovered a type of organelle, a fundamental cellular structure, that can turn nitrogen gas into a form that is useful for cell growth.
The discovery of the structure, called a nitroplast, in algae could bolster efforts to genetically engineer plants to convert, or ‘fix’, their own nitrogen, which could boost crop yields and reduce the need for fertilizers. The work was published in Science on 11 April1.
New cellular ‘organelle’ discovered inside fruit-fly intestines
“The textbooks say nitrogen fixation only occurs in bacteria and archaea,” says ocean ecologist Jonathan Zehr at the University of California, Santa Cruz, a co-author of the study. This species of algae is the “first nitrogen-fixing eukaryote”, he adds, referring to the group of organisms that includes plants and animals.
In 2012, Zehr and his colleagues reported that the marine algae Braarudosphaera bigelowii interacted closely with a bacterium called UCYN-A that seemed to live in, or on, the algal cells2. The researchers hypothesised that UCYN-A converts nitrogen gas into compounds that the algae use to grow, such as ammonia. In return, the bacteria were thought to gain a carbon-based energy source from the algae.
But in the latest study, Zehr and his colleagues conclude that UCYN-A should be classed as organelles inside the algae, rather than as a separate organism. According to genetic analysis from a previous study, ancestors of the algae and bacteria entered a symbiotic relationship around 100 million years ago, says Zehr. Eventually, this gave rise to the nitroplast organelle, now seen in B. bigelowii.
Defining organelles
Researchers use two key criteria to decide whether a bacterial cell has become an organelle in a host cell. First, the cell structure in question must be passed down through generations of the host cell. Second, the structure must be reliant on proteins provided by the host cell.
By imaging dozens of algae cells at various stages of cell division, the team found that the nitroplast splits in two just before the whole algae cell divides. In this way, one nitroplast is passed down from the parent cell to its offspring, as happens with other cell structures.
A seagrass harbours a nitrogen-fixing bacterial partner
Next, the researchers found that the nitroplast gets the proteins it needs to grow from the wider algae cell. The nitroplast itself — which makes up more than 8% of the volume of each host cell — lacks key proteins required for photosynthesis and making genetic material, says Zehr. “A lot of these proteins [from the algae] are just filling those gaps in metabolism,” he says.
The discovery was made possible thanks to work by study author Kyoko Hagino at Kochi University in Japan, who spent around a decade fine-tuning a way to grow the algae in the lab — which allowed it to be studied in more detail, says Zehr.
“It’s quite remarkable,” says Siv Andersson, who studies how organelles evolve at Uppsala University in Sweden. “They really see all these hallmarks that we think are characteristic of organelles.”
Upgraded plants
Understanding how the nitroplast interacts with its host cell could support efforts to engineer crops that can fix their own nitrogen, says Zehr. This would reduce the need for nitrogen-based fertilizers and avoid some of the environmental damage they cause. “The tricks that are involved in making this system work could be used in engineering land plants,” he says.
“Crop yields are majorly limited by availability of nitrogen,” says Eva Nowack, who studies symbiotic bacteria at the Heinrich Heine University Düsseldorf in Germany. “Having a nitrogen-fixing organelle in a crop plant would be, of course, fantastic.” But introducing this ability into plants will be no easy feat, she warns. Plant cells containing the genetic code for the nitroplast would need to be engineered in such a way that the genes were transferred stably from generation to generation, for example. “That would be the most difficult thing to do,” she says.
“It’s both a pleasure and very impressive to see this work build up to what is certainly a major stepping stone in understanding,” says Jeffrey Elhai, a cell biologist at Virginia Commonwealth University in Richmond, Vriginia.