Culture in animals can be broadly conceptualized as the sum of a population’s behavioural traditions, which, in turn, are defined as behaviours that are transmitted through social learning and that persist in a population over time4. Although culture was once thought to be exclusive to humans and a key explanation of our own evolutionary success, the existence of non-human cultures that change over time is no longer controversial. Changes in the songs of Savannah sparrows5 and humpback whales6,7,8 have been documented over decades. The sweet-potato-washing behaviour of Japanese macaques has also undergone several distinctive modifications since its inception at the hands of ‘Imo’, a juvenile female, in 19539. Imo’s initial behaviour involved dipping a potato in a freshwater stream and wiping sand off with her spare hand, but within a decade it had evolved to include repeated washing in seawater in between bites rather than in fresh water, potentially to enhance the flavour of the potato. By the 1980s, a range of variations had appeared among macaques, including stealing already-washed potatoes from conspecifics, and digging new pools in secluded areas to wash potatoes without being seen by scroungers9,10,11. Likewise, the ‘wide’, ‘narrow’ and ‘stepped’ designs of pandanus tools, which are fashioned from torn leaves by New Caledonian crows and used to fish grubs from logs, seem to have diverged from a single point of origin12. In this manner, cultural evolution can result in both the accumulation of novel traditions, and the accumulation of modifications to these traditions in turn. However, the limitations of non-human cultural evolution remain a subject of debate.
It is clearly true that humans are a uniquely encultured species. Almost everything we do relies on knowledge or technology that has taken many generations to build. No one human being could possibly manage, within their own lifetime, to split the atom by themselves from scratch. They could not even conceive of doing so without centuries of accumulated scientific knowledge. The existence of this so-called cumulative culture was thought to rely on the ‘ratchet’ concept, whereby traditions are retained in a population with sufficient fidelity to allow improvements to accumulate1,2,3. This was argued to require so-called higher-order forms of social learning, such as imitative copying13 or teaching14, which have, in turn, been argued to be exclusive to humans (although, see a review of imitative copying in animals15 for potential examples). But if we strip the definition of cumulative culture back to its bare bones, for a behavioural tradition to be considered cumulative, it must fulfil a set of core requirements1. In short, a beneficial innovation or modification to a behaviour must be socially transmitted among individuals of a population. This process may then occur repeatedly, leading to sequential improvements or elaborations. According to these criteria, there is evidence that some animals are capable of forming a cumulative culture in certain contexts and circumstances1,16,17. For example, when pairs of pigeons were tasked with making repeated flights home from a novel location, they found more efficient routes more quickly when members of these pairs were progressively swapped out, when compared with pairs of fixed composition or solo individuals16. This was thought to be due to ‘innovations’ made by the new individuals, resulting in incremental improvements in route efficiency. However, the end state of the behaviour in this case could, in theory, have been arrived at by a single individual1. It remains unclear whether modifications can accumulate to the point at which the final behaviour is too complex for any individual to innovate itself, but can still be acquired by that same individual through social learning from a knowledgeable conspecific. This threshold, often including the stipulation that re-innovation must be impossible within an individual’s own lifetime, is argued by some to represent a fundamental difference between human and non-human cognition3,13,18.
Bumblebees (Bombus terrestris) are social insects that have been shown to be capable of acquiring complex, non-natural behaviours through social learning in a laboratory setting, such as string-pulling19 and ball-rolling to gain rewards20. In the latter case, they were even able to improve on the behaviour of their original demonstrator. More recently, when challenged with a two-option puzzle-box task and a paradigm allowing learning to diffuse across a population (a gold standard of cultural transmission experiments21, as used previously in wild great tits22), bumblebees were found to acquire and maintain arbitrary variants of this behaviour from trained demonstrators23. However, these previous investigations involved the acquisition of a behaviour that each bee could also have innovated independently. Indeed, some naive individuals were able to open the puzzle box, pull strings and roll balls without demonstrators19,20,23. Thus, to determine whether bumblebees could acquire a behaviour through social learning that they could not innovate independently, we developed a novel two-step puzzle box (Fig. 1a). This design was informed by a lockbox task that was developed to assess problem solving in Goffin’s cockatoos24. Here, cockatoos were challenged to open a box that was sealed with five inter-connected ‘locks’ that had to be opened sequentially, with no reward for opening any but the final lock. Our hypothesis was that this degree of temporal and spatial separation between performing the first step of the behaviour and the reward would make it very difficult, if not impossible, for a naive bumblebee to form a lasting association between this necessary initial action and the final reward. Even if a bee opened the two-step box independently through repeated, non-directed probing, as observed with our previous box23, if no association formed between the combination of the two pushing behaviours and the reward, this behaviour would be unlikely to be incorporated into an individual’s repertoire. If, however, a bee was able to learn this multi-step box-opening behaviour when exposed to a skilled demonstrator, this would suggest that bumblebees can acquire behaviours socially that lie beyond their capacity for individual innovation.
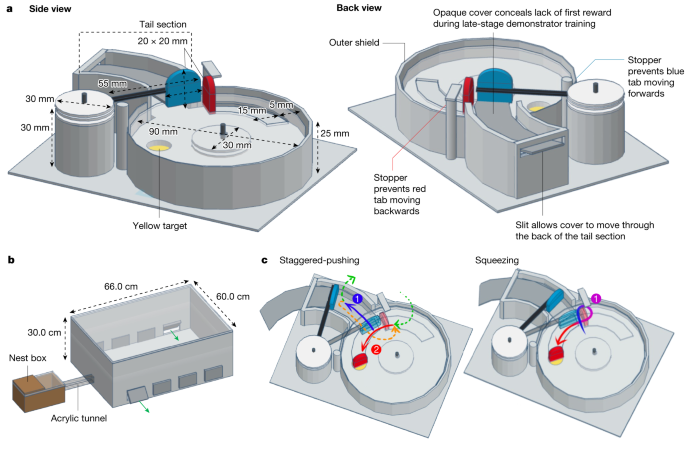
a, Puzzle-box design. Box bases were 3D-printed to ensure consistency. The reward (50% w/w sucrose solution, placed on a yellow target) was inaccessible unless the red tab was pushed, rotating the lid anti-clockwise around a central axis, and the red tab could not move unless the blue tab was first pushed out of its path. See Supplementary Information for a full description of the box design elements. b, Experimental set-up. The flight arena was connected to the nest box with an acrylic tunnel, and flaps cut into the side allowed the removal and replacement of puzzle boxes during the experiment. The sides were lined with bristles to prevent bees escaping. c, Alternative action patterns for opening the box. The staggered-pushing technique is characterized by two distinct pushes (1, blue arrow and 2, red arrow), divided by either flying (green arrows) or walking in a loop around the inner side of the red tab (orange arrow). The squeezing technique is characterized by a single, unbroken movement, starting at the point at which the blue and red tabs meet and pushing through, squeezing between the outer side of the red tab and the outer shield, and making a tight turn to push against the red tab.
The two-step puzzle box (Fig. 1a) relied on the same principles as our previous single-step, two-option puzzle box23. To access a sucrose-solution reward, placed on a yellow target, a blue tab had to first be pushed out of the path of a red tab, which could then be pushed in turn to rotate a clear lid around a central axis. Once rotated far enough, the reward would be exposed beneath the red tab. A sample video of a trained demonstrator opening the two-step box is available (Supplementary Video 1). Our experiments were conducted in a specially constructed flight arena, attached to a colony’s nest box, in which all bees that were not currently undergoing training or testing were confined (Fig. 1b).
In our previous study, several bees successfully learned to open the two-option, single-step box during control population experiments, which were conducted in the absence of a trained demonstrator across 6–12 days23. Thus, to determine whether the two-step box could be opened by individual bees starting from scratch, we sought to conduct a similar experiment. Two colonies (C1 and C2) took part in these control population experiments for 12 days, and one colony (C3) for 24 days. In brief, on 12 or 24 consecutive days, bees were exposed to open two-step puzzle boxes for 30 min pre-training and then to closed boxes for 3 h (meaning that colonies C1 and 2 were exposed to closed boxes for 36 h total, and colony C3 for 72 h total). No trained demonstrator was added to any group. On each day, bees foraged willingly during the pre-training, but no boxes were opened in either colony during the experiment. Although some bees were observed to probe around the components of the closed boxes with their proboscises, particularly in the early population-experiment sessions, this behaviour generally decreased as the experiment progressed. A single blue tab was opened in full in colony C1, but this behaviour was neither expanded on nor repeated.
Learning to open the two-step box was not trivial for our demonstrators, with the finalized training protocol taking around two days for them to complete (compared with several hours for our previous two-option, single-step box23). Developing a training protocol was also challenging. Bees readily learned to push the rewarded red tab, but not the unrewarded blue tab, which they would not manipulate at all. Instead, they would repeatedly push against the blocked red tab before giving up. This necessitated the addition of a temporary yellow target and reward beneath the blue tab, which, in turn, required the addition of the extended tail section (as seen in Fig. 1a), because during later stages of training this temporary target had to be removed and its absence concealed. This had to be done gradually and in combination with an increased reward on the final target, because bees quickly lost their motivation to open any more boxes otherwise. Frequently, reluctant bees had to be coaxed back to participation by providing them with fully opened lids that they did not need to push at all. In short, bees seemed generally unwilling to perform actions that were not directly linked to a reward, or that were no longer being rewarded. Notably, when opening two-step boxes after learning, demonstrators frequently pushed against the red tab before attempting to push the blue, even though they were able to perform the complete behaviour (and subsequently did so). The combination of having to move away from a visible reward and take a non-direct route, and the lack of any reward in exchange for this behaviour, suggests that two-step box-opening would be very difficult, if not impossible, for a naive bumblebee to discover and learn for itself—in line with the results of the control population experiment.
For the dyad experiments, a pair of bees, including one trained demonstrator and one naive observer, was allowed to forage on three closed puzzle boxes (each filled with 20 μl 50% w/w sucrose solution) for 30–40 sessions, with unrewarded learning tests given to the observer in isolation after 30, 35 and 40 joint sessions. With each session lasting a maximum of 20 min, this meant that observers could be exposed to the boxes and the demonstrator for a total of 800 min, or 13.3 h (markedly less time than the bees in the control population experiments, who had access to the boxes in the absence of a demonstrator for 36 or 72 h total). If an observer passed a learning test, it immediately proceeded to 10 solo foraging sessions in the absence of the demonstrator. The 15 demonstrator and observer combinations used for the dyad experiments are listed in Table 1, and some demonstrators were used for multiple observers. Of the 15 observers, 5 passed the unrewarded learning test, with 3 of these doing so on the first attempt and the remaining 2 on the third. This relatively low number reflected the difficulty of the task, but the fact that any observers acquired two-step box-opening at all confirmed that this behaviour could be socially learned.
The post-learning solo foraging sessions were designed to further test observers’ acquisition of two-step box-opening. Each session lasted up to 10 min, but 50 μl 50% sucrose solution was placed on the yellow target in each box: as Bombus terrestris foragers have been found to collect 60–150 μl sucrose solution per foraging trip depending on their size, this meant that each bee could reasonably be expected to open two boxes per session25. Although all bees who proceeded to the solo foraging stage repeated two-step box-opening, confirming their status as learners, only two individuals (A-24 and A-6; Table 1) met the criterion to be classified as proficient learners (that is, they opened 10 or more boxes). This was the same threshold applied to learners in our previous work with the single-step two-option box23. However, it should be noted that learners from our present study had comparatively limited post-learning exposure to the boxes (a total of 100 min on one day) compared with those from our previous work. Proficient learners from our single-step puzzle-box experiments typically attained proficiency over several days of foraging, and had access to boxes for 180 min each day for 6–12 days23. Thus, these comparatively low numbers of proficient bees are perhaps unsurprising.
Two different methods of opening the two-step puzzle box were observed among the trained demonstrators during the dyad experiments, and were termed ‘staggered-pushing’ and ‘squeezing’ (Fig. 1c; Supplementary Video 2). This finding essentially transformed the experiment into a ‘two-action’-type design, reminiscent of our previous single-step, two-option puzzle-box task23. Of these techniques, squeezing typically resulted in the blue tab being pushed less far than staggered-pushing did, often only just enough to free the red tab, and the red tab often shifted forward as the bee squeezed between this and the outer shield. Among demonstrators, the squeezing technique was more common, being adopted as the main technique by 6 out of 9 individuals (Table 1). Thus, 10 out of 15 observers were paired with a squeezing demonstrator.
Although not all observers that were paired with squeezing demonstrators learned to open the two-step box (5 out of 10 succeeded), all observers paired with staggered-pushing demonstrators (n = 5) failed to learn two-step box-opening. This discrepancy was not due to the number of demonstrations being received by the observers: there was no difference in the number of boxes opened by squeezing demonstrators compared with staggered-pushing demonstrators when the number of joint sessions was accounted for (unpaired t-test, t = −2.015, P = 0.065, degrees of freedom (df) = 13, 95% confidence interval (CI) = −3.63–0.13; Table 2). This might have been because the squeezing demonstrators often performed their squeezing action several times, looping around the red tab, which lengthened the total duration of the behaviour despite the blue tab being pushed less than during staggered-pushing. Closer investigation of the dyads that involved only squeezing demonstrators revealed that demonstrators paired with observers that failed to learn tended to open fewer boxes, but this difference was not significant. There was also no difference between these dyads and those that included a staggered-pushing demonstrator (one-way ANOVA, F = 2.446, P = 0.129, df = 12; Table 2 and Fig. 2a). Together, these findings suggested that demonstrator technique might influence whether the transmission of two-step box-opening was successful. Notably, successful learners also appeared to acquire the specific technique used by their demonstrator: in all cases, this was the squeezing technique. In the solo foraging sessions recorded for successful learners, they also tended to preferentially adopt the squeezing technique (Table 1). The potential effect of certain demonstrators being used for multiple dyads is analysed and discussed in the Supplementary Results (see Supplementary Table 2 and Supplementary Fig. 4).
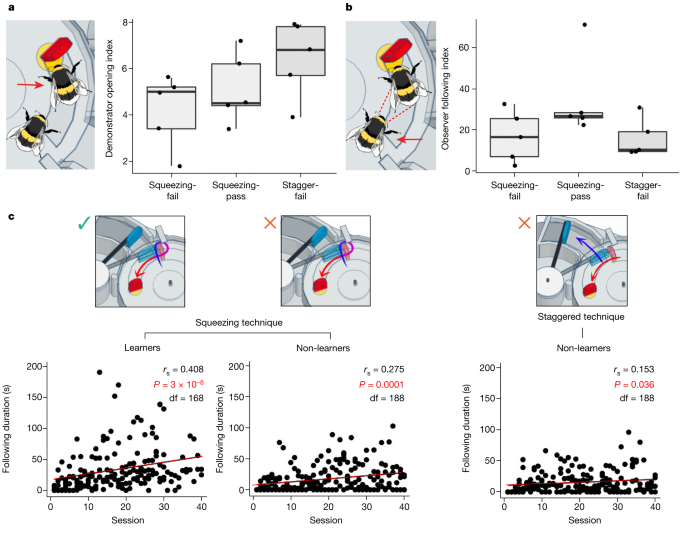
a, Demonstrator opening index. The demonstrator opening index was calculated for each dyad as the total incidence of box-opening by the demonstrator/number of joint foraging sessions. b, Observer following index. Following behaviour was defined as the observer being present on the surface of the box, within a bee’s length of the demonstrator, while the demonstrator performed box-opening. The observer following index was calculated as the total duration of following behaviour/number of joint foraging sessions. Data in a,b were analysed using one-way ANOVA and are presented as box plots. The bounds of the box are drawn from quartile 1 to quartile 3 (showing the interquartile range), the horizontal line within shows the median value and the whiskers extend to the most extreme data point that is no more than 1.5 × the interquartile range from the edge of the box. n = 15 independent experiments (squeezing-pass group, n = 5; squeezing-fail group, n = 5; and staggered-pushing-fail (stagger-fail) group, n = 5). c, Duration of following behaviour over the dyad joint foraging sessions. Following behaviour significantly increased with the number of joint foraging sessions, with the sharpest increase seen in dyads that included a squeezing demonstrator and an observer that successfully acquired two-step box-opening. Data were analysed using Spearman’s rank correlation coefficient tests (two-tailed), and the figures show measures taken from each observer in each group. Data for individual observers are presented in Supplementary Fig. 1.
To determine whether observer behaviour might have differed between those who passed and failed, we investigated the duration of their ‘following’ behaviour, which was a distinctive behaviour that we identified during the joint foraging sessions. Here, an observer followed closely behind the demonstrator as it walked on the surface of the box, often close enough to make contact with the demonstrator’s body with its antennae (Supplementary Video 3). In the case of squeezing demonstrators, which often made several loops around the red tab, a following observer would make these loops also. To ensure we quantified only the most relevant behaviour, we defined following behaviour as ‘instances in which an observer was present on the box surface, within a single bee’s length of the demonstrator, while it performed two-step box-opening’. Thus, following behaviour could be recorded only after the demonstrator began to push the blue tab, and before it accessed the reward. This was quantified for each joint foraging session for the dyad experiments (Supplementary Table 1). There was no significant correlation between the demonstrator opening index and the observer following index (Spearman’s rank correlation coefficient, rs = 0.173, df = 13, P = 0.537; Supplementary Fig. 2), suggesting that increases in following behaviour were not due simply to there being more demonstrations of two-step box-opening available to the observer.
There was no statistically significant difference in the following index between dyads with squeezing and dyads with staggered-pushing demonstrators; between dyads in which observers passed and those in which they failed; or when both demonstrator preference and learning outcome were accounted for (Table 2). This might have been due to the limited sample size. However, the following index tended to be higher in dyads in which the observer successfully acquired two-step box-opening than in those in which the observer failed (34.82 versus 16.26, respectively; Table 2) and in dyads with squeezing demonstrators compared with staggered-pushing demonstrators (25.78 versus 15.76, respectively; Table 2). When both factors were accounted for, following behaviour was most frequent in dyads with a squeezing demonstrator and an observer that successfully acquired two-step box-opening (34.82 versus 16.75 (‘squeezing-fail’ group) versus 15.76 (‘staggered-pushing-fail’ group); Table 2).
There was, however, a strong positive correlation between the duration of following behaviour and the number of joint foraging sessions, which equated to time spent foraging alongside the demonstrator. This association was present in dyads from all three groups but was strongest in the squeezing-pass group (Spearman’s rank order correlation coefficient, rs = 0.408, df = 168, P < 0.001; Fig. 2c). This suggests, in general, either that the latency between the start of the demonstration and the observer following behaviour decreased over time, or that observers continued to follow for longer once arriving. However, the observers from the squeezing-pass group tended to follow for longer than any other group, and the duration of their following increased more rapidly. This indicates that following a conspecific demonstrator as it performed two-step box-opening (and, specifically, through squeezing) was important to the acquisition of this behaviour by an observer.